Background
Mature T-cell lymphoma (TCL), an aggressive and heterogenous group of T cell neoplasms, constitutes less than 15% of adult non-Hodgkin lymphomas. CD5 is an inhibitory T cell receptor molecule expressed on mature T cells. CD5 expression has been associated with worse outcomes in B-cell malignancies, however this association has not been explored in TCL. Furthermore, CD5 has become a promising immunotherapeutic target for TCL. We herein aimed to characterize CD5 expression in TCL subtypes at diagnosis and relapse, and its association with clinical features and outcomes in a single center cohort.
Methods
We retrospectively identified 109 patients (pts) diagnosed with non-cutaneous and non-leukemic mature TCL, with data on CD5 expression on tumor tissue by immunohistochemistry (IHC) and/or flow cytometry (FC), from the electronic medical records at the Hospital of the University of Pennsylvania (HUP). CD5 positivity (CD5+) was defined as any percentage of CD5+ malignant cells on FC and/or IHC. Chi-square test was used to compare baseline pt characteristics at the initial presentation between CD5 expression groups. Treatment response (TR) was defined as achieving complete or partial response by the end of initial treatment. Logistic regression models were used to assess the impact of clinical parameters on the overall TR rate and odds ratios (OR) were computed. Cox proportional hazards models were used to evaluate the effects of clinical parameters on overall survival (OS) and progression-free survival (PFS) and were reported as hazard ratios (HR). We also conducted Kaplan-Meier (KM) analyses for OS and PFS by CD5 expression status. The OR and HR are reported with 95% CI in brackets. P-values <0.05 were considered significant. All analyses were conducted in SAS version 9.4 (SAS Institute Inc).
Results
We collected and evaluated data from a total of 109 adult pts diagnosed with TCL at HUP between 1/1/05-1/1/21. Median age at diagnosis was 58 (range: 19 - 86 years); 56% were men; the most common diagnosis was ALCL (n=37) followed by nodal TFH lymphoma (n=29), PTCL-NOS (n=16), ENKTCL (n=13) and other extranodal (EN) TCL (n=14) (Table.1). Compared to CD5- pts, a higher percentage of CD5+ pts were diagnosed at age >60 (CD5-:34.7%, CD5+:55.0%, p=0.0343), and stage 3 or 4 (CD5-:46.9%, CD5+:70%, p=0.0142), but fewer CD5+ pts had EN involvement at diagnosis (CD5-:77.6%, CD5+:50%, p=0.0031). CD5 was also differentially expressed by TCL subtypes: nearly all TFH TCL (93.1%) and PTCL-NOS expressed CD5 (87.5%), whereas CD5 was less commonly expressed in ALCL (39.4%,), other ENTCL (27.8%), and ENKTCL (7.7%) (p<0.0001). In addition, on univariate analysis (UVA), CD5 expression was associated with stage 3 or 4 (p=0.014), age >60 (p=0.034), and less common EN involvement (p=0.031), but not with treatment response, relapse or survival. On UVA for treatment response, established risk factors such as ≥ 2 EN involvement (OR=0.25 [0.09, 0.6], p=0.0243) and high LDH (OR=0.26 [0.08, 0.85], p=0.0267), were shown to be negative predictors. Multivariate analysis (MVA) for response also predicted high LDH as a negative predictor (OR=0.29 [0.08,0.99], p=0.0477). CD5 expression was not associated with treatment response on either UVA (OR=1.18 [0.50, 2.78], p=0.7042) or MVA (OR=1.00 [0.33,3.60], p=0.9969).
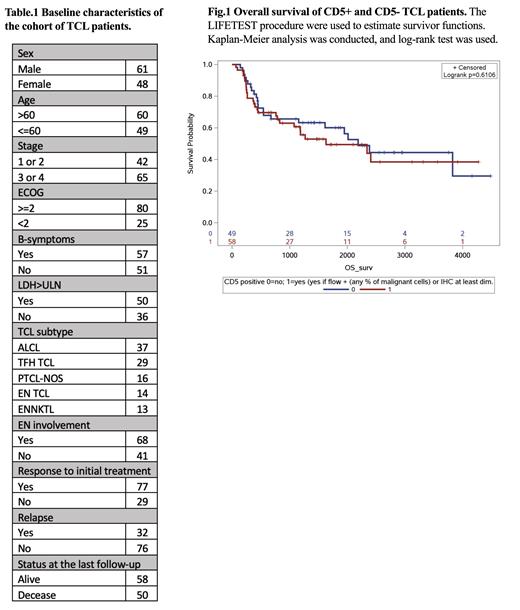
On UVA, CD5 positivity did not impact OS (HR=1.2 [0.7,2.0], p=0.6118) nor PFS (HR=1.5 [0.7,3.0], p=0.2862), while worse outcomes were associated with established risk factors, including ECOG performance status ≥ 2 (OS HR=2.0 [1.0-3.8], p=0.0440), ≥2 EN involvement (OS HR=3.1 [1.7-5.7], p=0.0002; PFS HR 13.1 [2.9-59.9], p=0.0009), and B symptoms (OS HR=2.1 [1.2-3.7], p=0.0117). TFH TCL was also found to be associated with worse OS (HR= 2.6 [1.3-5.5], p=0.0101). In our cohort of 35 pts with CD5 expression data at relapses, 100% pts remained CD5+ (n=19), 68.75% pts remained CD5- (n=11), but 31.25% pts gained CD5 expression (n=5), and none lost CD5 expression. Based on KM analyses, CD5 expression did not impact PFS or OS (Fig. 1).
Conclusions
In this cohort of 109 TCL pts, CD5 expression was common in TFH TCL, PTCL-NOS, ALCL, and ENTCL, and was retained or gained at relapse, thus supporting its potential as a therapeutic target. CD5 expression was associated with advanced age and stage, while EN involvement was less common. CD5 expression was not associated with a differential treatment response, PFS, nor OS.
Disclosures
Chong:MJH Healthcare Holdings, LLC: Honoraria; Genentech: Research Funding; Abbvie: Research Funding; Novartis: Honoraria; BMS: Honoraria; Beigene: Honoraria. Ruella:viTToria biotherapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other: Scientific Founder, Research Funding; Bristol Myers Squibb: Consultancy; NanoString: Consultancy, Research Funding; AbClon: Consultancy, Research Funding; Bayer: Consultancy; Beckman Coulter: Research Funding; GlaxoSmithKline: Consultancy. Landsburg:ADCT: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Epizyme: Membership on an entity's Board of Directors or advisory committees; Calithera: Membership on an entity's Board of Directors or advisory committees, Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Curis: Research Funding; Novartis: Membership on an entity's Board of Directors or advisory committees, Other: Travel funding; Morphosys: Membership on an entity's Board of Directors or advisory committees. Nasta:Merck-Data Safety Monitoring Committee: Membership on an entity's Board of Directors or advisory committees; Ono Pharmaceutical: Research Funding; ADC Therapeutics: Honoraria; Pharmacyclics: Research Funding; Genentech/Roche: Research Funding; Loxo/Lilly: Research Funding; Accrotech: Honoraria; Millennium Takeda: Research Funding; Raphael: Research Funding. Svoboda:Genmab: Consultancy; Incyte: Consultancy, Research Funding; ADCT: Consultancy; TG Therapeutics: Research Funding; Atara: Consultancy; BMS: Consultancy, Research Funding; Astra Zeneca: Consultancy, Research Funding; Adaptive: Consultancy, Research Funding; Merck: Research Funding; Pharmacyclics: Consultancy, Research Funding; SEAGEN: Consultancy, Research Funding. Barta:Daiichi Sankyo: Consultancy; Acrotech: Consultancy; Janssen: Consultancy; Affimed: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal